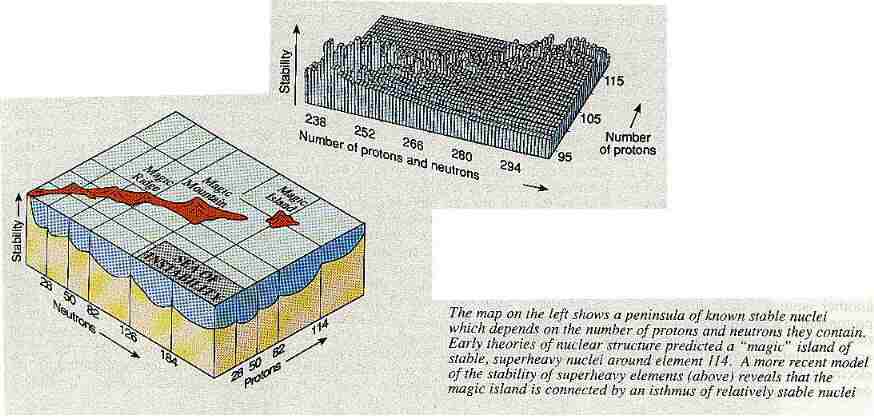
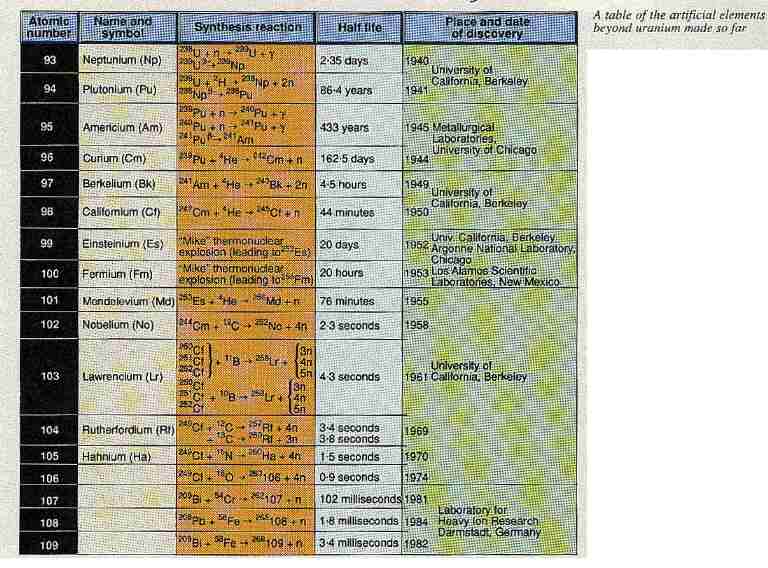
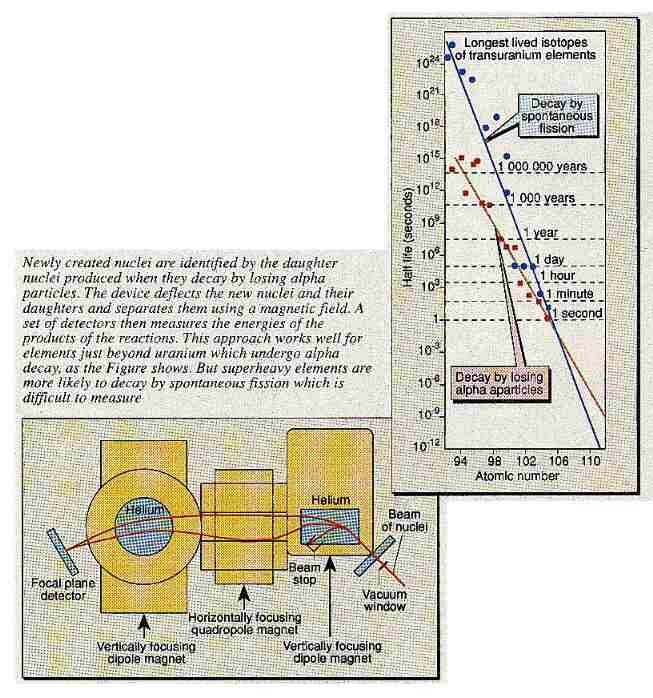
One of the curious things about modern space fiction films is that while they try to get the astronomy and physics reasonably correct, the scriptwriters often completely ignore the rules of chemical structure. For example, Captain Kirk in Star Trek finds an alien spaceship whose hull is made of some unknown element with bizarre properties. Any chemist knows that such a discovery is just about impossible because every stable element that can exist in the Universe is already known.
But that may not be completely true. Over the past two or three decades, American, German and Soviet research groups have been trying to create artificial elements that are extremely heavy. The heaviest elements made so far are not very stable and, therefore, are extremely radioactive but some theorists think that it might be possible to make even heavier elements that are more stable.
An element consists of atoms, each of which has a nucleus with a characteristic number of positively charged protons, defined by the atomic number, and a certain number of neutrons which stabilise the nucleus by ‘diluting’ the repulsive electrical charge between the protons. Most elements come in several varieties called isotopes which have differing numbers of neutrons. The total number of protons and neutrons are denoted by the atomic mass number (for example, carbon-12 has six protons and six neutrons). The lightest element hydrogen, contains atoms with a nucleus of one proton, but another isotope called deuterium contains nuclei with one proton and one neutron, while a third isotope, tritium, contains two neutrons and a proton. This isotope is unstable, or radioactive, disintegrating to give off beta…