A record number of reagents has been made to combine in a single vessel in a reaction scheme devised by a group of German chemists. Because it reduces the need to separate and purify intermediate products, such a scheme could simplify the manufacture of drugs and agrochemicals.
To make complicated molecules, chemists usually have to use a sequence of several separate reactions. In each step, a pair of reagents yields a product, which must be isolated and purified to remove by-products along with any starting material that has remained. The purified intermediate product then becomes one of the reagents in the next step.
However, in what are known as ‘one-pot’ reactions, chemists simply throw three or more ingredients into a reaction vessel. Under appropriate conditions of pressure, temperature and pH, the components then form the desired product.
Advertisement
Alexander Domling and Ivar Ugi of the University of Munich have raised this technique to new heights. Not for them a one-pot reaction with a mere three or four components: they have devised a reaction involving seven reagents. They say such a system should make it is possible to build a molecule with many chemical groups without isolating the intermediate products.
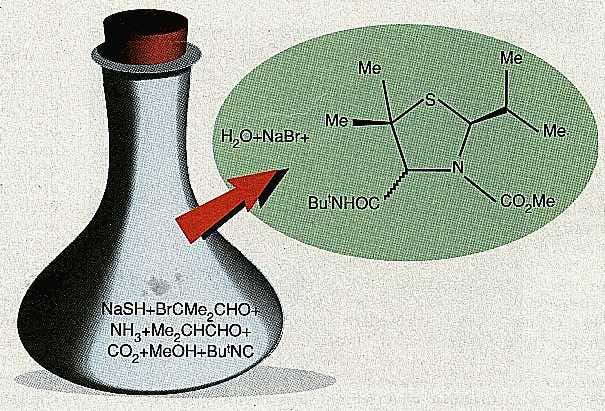
In the first step of Domling and Ugi’s reaction (see figure), sodium hydrogen sulphide (NaSH) and α-bromoisobutyraldehyde (BrCMe2CHO) reacted to produce HSCMe2CHO. This then reacted with ammonia (NH3) and isobutyraldehyde (Me2CHCHO) to produce a ‘condensation compound’.
Meanwhile, carbon dioxide (CO2) was reacting with methanol (MeOH) to form MeOCO2H, which transferred a proton to the condensation compound. The protonated compound then reacted with MeOCO2– and tert-butylisocyanide (ButNC). In the final step, the product of this reaction rearranged itself and formed the product with a yield of 45 per cent (Angewandte Chemie, International Edition, vol 4, p 563).FIG-mg18803201.jpg